 Proud to announce the publication of Xu et al., Magn Reson 2021, “Fragile protein folds: Sequence and environmental factors affecting the equilibrium of two interconverting, stably folded protein conformations” in a special collection celebrating the 80th birthday of our NMR colleague Rob Kaptein.
Proud to announce the publication of Xu et al., Magn Reson 2021, “Fragile protein folds: Sequence and environmental factors affecting the equilibrium of two interconverting, stably folded protein conformations” in a special collection celebrating the 80th birthday of our NMR colleague Rob Kaptein.
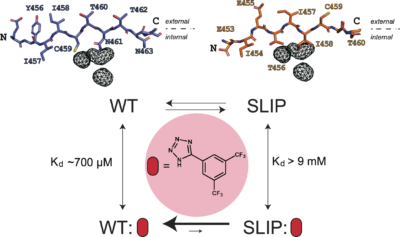
This work extends our studies of the ARNT PAS-B Y456T model system for β-strand slipping, initially established over ten years ago in the lab (Evans et al. PNAS 2009; Evans & Gardner JACS 2009). In the early days of this project, we fortuitously found several point mutations could affect the equilibrium of the WT and SLIP conformers that differ solely by a change in strand register. Some of these mutations worked together combinatorially, but we really wanted more insight into mechanism. We also really wanted a way to control this equilibrium on the fly – in the early days, this was only done by chromatographically-separating one conformer from the other in the cold, then running down the hall to throw a sample in the NMR to watch the equilibrium re-establish in real time. We’ve recently reported that this can be done by applying high pressure (Xu et al., Biophys J 2021), but given the ability to target PAS domains with small molecules, it seemed like we really should be able to find ways to shift this equilibrium by simply adding a compound to a tube.
Our manuscript addresses both of these topics with a collection of results. Mechanistically, we’ve been able to demonstrate that point mutations which impact the equilibrium of two folded state structures do so with particular effects on the unfolded state that we think is visited as part of the interconversion process between the two folded forms. Control-wise, we’ve shown that compounds which selectively bind one of the conformers can set up a coupled equilibrium, shifting the protein into the ligand-binding form. We also checked into some other ways to shift the equilibrium – everything from pH and salt to loop insertions; some of these work better than others, but we’re again seeing some neat ways that we might be able to control protein conformation and function in the future.
I’ll close by noting that I’m really proud of this work on multiple levels – it links the Texan and New Yorker iterations of the group, includes a CCNY undergrad [Leandro] sharing some of his undergrad honors dissertation work, and gives me a chance to tip my hat to Rob to thank him for great work in the PAS and photoreceptor field in their studies of PYP (and great hospitality on some visits to Utrecht early in my independent career).
