 Proud to announce the publication of Xu et al., Biophys J 2021, “Volume and Compressibility Differences Between Protein Conformations Revealed by High-Pressure NMR,” along with a great accompanying New & Notable overview from Remco Sprangers.
Proud to announce the publication of Xu et al., Biophys J 2021, “Volume and Compressibility Differences Between Protein Conformations Revealed by High-Pressure NMR,” along with a great accompanying New & Notable overview from Remco Sprangers.
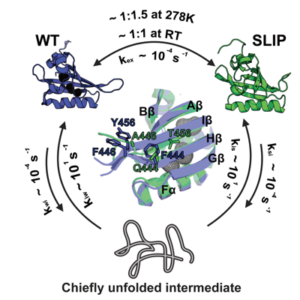
This work returns us to a neat model system for metamorphic proteins which we uncovered over a decade ago, the Y456T point mutant of the ARNT PAS-B domain (Evans et al. PNAS 2009; Evans & Gardner JACS 2009). Such metamorphic proteins break one of the fundamental rules of protein structure by being able to adopt two fundamentally different structures with one sequence; for this ARNT PAS-B variant, the two structures differ in the location of an important beta-strand. Our prior work showed what those two structures are and noted that real-time interconversion – which occurs on the timescale of hours – probably requires unfolding the protein to a chiefly-denatured intermediate.
Here we explore this process further, using solution NMR and hydrostatic pressure to shift the equilibrium in real time between the two conformations. In doing so, we’ve gotten quantitative insights into the mechanical properties of both end states and the short-lived intermediate that’s otherwise difficult to characterize. We think that this teaches us a bit about the drivers for this equilibrium and how we might be able to control it, as we mention here and in another Xu et al. paper (Xu et al., Magn Reson 2021) that followed this one quickly afterwards.
Quick KG Lab trivia note: All four authors are either native-born Canadians or worked there for various stages of their career, representing the fine provinces of ON, QC, BC, and AB in the process. True North = True Magnetic North…?
